Safe Apnoea Time
PREOX
REOX
APOX
CONOX
Safe Apnoea Time
PREOX
REOX
APOX
CONOX
the oxygenation continuum
The primary goal of airway management is to maintain tissue oxygenation so as to prevent patients from suffering hypoxic injury. While techniques exist which allow airway management without the occurrence of apnoea/airway obstruction, these may not always be successful or clinically appropriate. Additionally, these techniques are usually only implemented when a challenging airway is anticipated, and as such, have limited utility in the management of the unanticipated challenging airway. Thus the risk of an interruption to alveolar oxygen delivery and subsequent hypoxic harm, is inherently involved with the process of airway management in both the elective and emergency situation.
When apnoea/obstruction occurs, oxygen delivery to the alveoli must be restored before critical desaturation occurs in order to avoid the risk of hypoxic injury. Two complementary strategies are available to preserve tissue oxygenation and reduce the risk of patients suffering hypoxic injury:
Establishing alveolar oxygen delivery: is the definitive strategy to preserve tissue oxygenation. A sustained interruption to alveolar oxygen delivery will eventually inevitably lead to decreases in blood oxygen content (“blood oxygenation”) and delivery of oxygen to the tissues (“tissue oxygenation”). Interruptions to tissue oxygenation which are sufficiently prolonged or severe will result in hypoxic injury to the patient. Establishment of alveolar oxygen delivery is represented in the Vortex Approach by entry into the Green Zone.
Extending the safe apnoea time: is a supplementary strategy that can prolong the time until blood and tissue oxygenation decreases in the event that alveolar oxygen delivery by ventilation/insufflation is transiently interrupted (safe apnoea time). If alveolar oxygen delivery is not restored, however, the patient will still eventually falls in blood and tissue oxygenation to dangerous levels. Extending the safe apnoea time merely delays critical desaturation and provides time to implement the definitive strategy that will ultimately prevent/reverse desaturation. It is thus an adjunct, not an alternative, to restoring alveolar oxygen delivery.
“Extending the safe apnoea time merely delays critical desaturation and provides time to implement the definitive airway strategy that will ultimately prevent/reverse desaturation. It is thus an adjunct, not an alternative, to restoring alveolar oxygen delivery.”
Whilst other factors such as gas exchange, haemoglobin concentration and blood flow may also impact tissue oxygenation, these are not influenced by the interventions associated with airway management. Thus it is important to recognise that maintenance of normal oxygen saturations is not synonymous with entry into the Green Zone. Normal oxygen saturations may be maintained within the safe apnoea time despite the absence of ongoing alveolar oxygen delivery. Conversely factors influencing gas exchange or cardiac output may lead to compromise of oxygen saturations despite the presence of alveolar oxygen delivery. Note that the term "alveolar oxygenation" has been avoided due to ambiguity over whether it refers to delivery of fresh oxygen to the alveoli (alveolar oxygen delivery) or maintenance of high alveolar concentrations (which may occur due to effective preoxygenation despite airway obstruction and an interruption to alveolar oxygen delivery).
The concept of the oxygenation continuum provides a visual representation describing the relationship between techniques to establish alveolar oxygen delivery and techniques to prolong the safe apnoea time in minimising the risk of exposing patients to hypoxic injury.
Under routine circumstances the time taken to establish entry into the Green Zone is shorter than the time for critical desaturation, providing a margin of safety for airway management during which the oxygenation continuum is maintained, avoiding exposing the patient to critical hypoxia. The actual time available until critical desaturation occurs if alveolar oxygen delivery is interrupted is only known retrospectively but there are a number of patient factors which might influence it including:
- Body habitus: obesity, pregnancy and other factors which decrease the size of the functional residual capacity may limit the safe apnoea time.
- Increased oxygen consumption: e.g. sepsis, pregnancy, paediatrics may decrease the safe apnoea time
- Impaired gas exchange: respiratory disease may decrease safe apnoea time
- Decreased blood oxygen carrying capacity: anaemia may decrease safe apnoea time
- Decreased cardiac output: may lead to increased oxygen extraction, lower mixed venous oxygen content, increased alveolar oxygen uptake and a lower oxygen content of any shunted blood - all of which will tend to depress the arterial oxygen saturations.
- Techniques to increase the safe apnoea time: preoxygenation, reoxygenation, apnoeic oxygenation, conserving oxygenation
Similarly the time taken to establish alveolar oxygen delivery is unknown prospectively but may be influenced by a number of patient, situational or clinician factors. These are outlined in relation to airway assessment.
If the safe apnoea time is shortened &/or the time to entry into the Green Zone prolonged, the margin of safety is reduced. If this occurs to a sufficient extent the oxygenation continuum may be interrupted, exposing the patient to critical hypoxia.
Minimising the risk of exposing the patient to prolonged hypoxia necessitates maximising the safe apnoea time and minimising the time to enter the Green Zone so as to optimise the margin of safety and decrease the chance of interruption to the oxygenation continuum.
- The Vortex Approach is directed at minimising the time to gain entry into the Green Zone by establishing alveolar oxygen delivery as efficiently as possible.
- Techniques to prolong the safe apnoea time maximise the time to critical desaturation. These techniques are not part of the Vortex Approach but are valuable complementary strategies that should be utilised with it in an integrated fashion. The information outlined below in relation to these techniques is based predominantly on the work or Scott Weingart, Richard Levitan, Anil Patel and Andrew Heard.
“Extending the safe apnoea time thus makes contributions to both technical and human factors considerations that can decrease the risk of patient hypoxia during airway management”
Delaying desaturation by extending the safe apnoea time not only reduces the risk of exposing the patient to hypoxia while airway interventions are performed but also makes an important psychological contribution, decreasing clinician stress and thereby potentially improving both cognitive and motor function during airway management. These improvements act synergistically with the principles of the Vortex Approach to augment the quality of any interventions by improving both decision making and technical skills and thus the likelihood of success at establishing alveolar oxygen delivery by either the upper airway lifelines or CICO Rescue. Extending the safe apnoea time thus makes contributions to both technical and human factors considerations that can decrease the risk of patient hypoxia during airway management.

Preoxygenation
PREOX
Preoxygenation
PREOX
Prinicples of preoxygenation
Preoxygenation is a safe, simple and effective technique to increase the safe apnoea time. Optimal preoxygenation requires attention to three key elements:
- Maximising the oxygen concentration in the lung functional residual capacity (FRC).
- Maximising the volume of the lung FRC
- Optimising gas exchange from the oxygenated alveoli
The first two elements increase the oxygen content in the FRC which can be used to sustain blood oxygenation in the event that alveolar oxygen delivery is interrupted. The third element improves uptake of alveolar oxygen into the blood and decreases shunt in order to maximise the blood oxygenation that can be achieved from these alveolar oxygen stores.
Alveolar oxygen concentration
Effective preoxygenation with 100% oxygen increases the oxygen content of gas in the patient’s FRC from 21% towards 100% which should theoretically produce a proportionate increase in the safe apnoea time. Whilst in practice achieving alveolar oxygen concentrations of 100% is not possible, increasing the end-tidal oxygen concentration to levels of 80-90% is usually readily achievable. The requirements for maximising alveolar oxygen concentration are as follows:
1. High Oxygen Concentration Device: despite being connected to a wall outlet or cylinder which provides a source of 100% oxygen, not all oxygen delivery devices are able to supply this 100% oxygen to the patient undiluted.
- Oxygen delivery devices such as an anaesthetic circuit (circle, Mapleson, etc) or some bag-valve-mask devices, are capable of providing close to 100% oxygen due to the presence of a reservoir bag and valves as well as a mask which can create a tight seal on the face. Together these factors prevent rebreathing/entrainment of room air allowing the device to deliver undiluted 100% oxygen to the patient.
- Whilst it is typically well appreciated that oxygen delivery devices such as non-rebreather masks, Hudson masks and nasal prongs are unable to deliver a high oxygen concentration to the patient, there is less awareness that even some designs of bag-valve-mask (BVM) device (those without an expiratory port valve), are able to deliver an oxygen concentrations of only 60% during spontaneous ventilation due to dilution of the 100% oxygen source by room air. During positive pressure ventilation BVM's will deliver 100% irrespective of whether they have an expiratory port valve. Thus BVM's without an expiratory port valve are suitable for reoxygenation of an apnoeic patient (positive pressure ventilation) but not preoxygenation (spontaneous ventilation).
- Even BVM's with an expiratory port valve may entrain room air if minute ventilation is high enough to exceed the flow of 100% oxygen supplying the device. This can lead to a dramatic fall in inspired oxygen concentration and occurs independently of whether the device is being used during spontaneous or positive pressure ventilation.
- Use of supplementary oxygen sources can compensate for the inability of some oxygen delivery devices to provide a closed system and increase the FiO2 provided by them towards acceptable levels for preoxygenation.
This video explains the reasons for the variability in the oxygen concentration provided by an array of devices and demonstrates the FiO2 achieved with them under a variety of conditions. These results are summarised in the accompanying table.
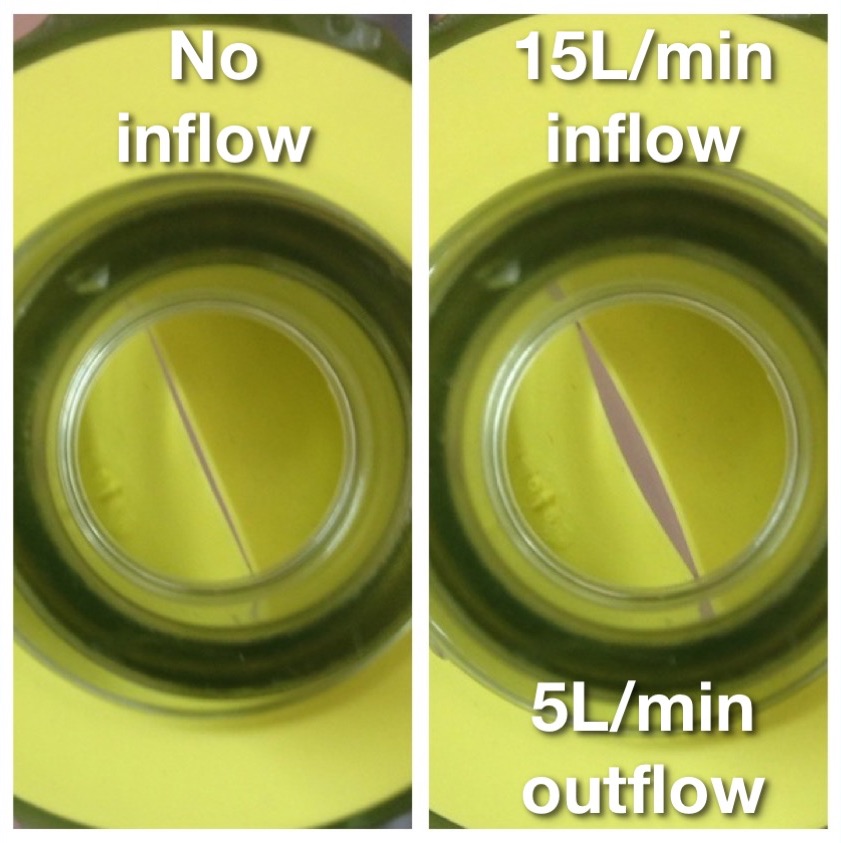
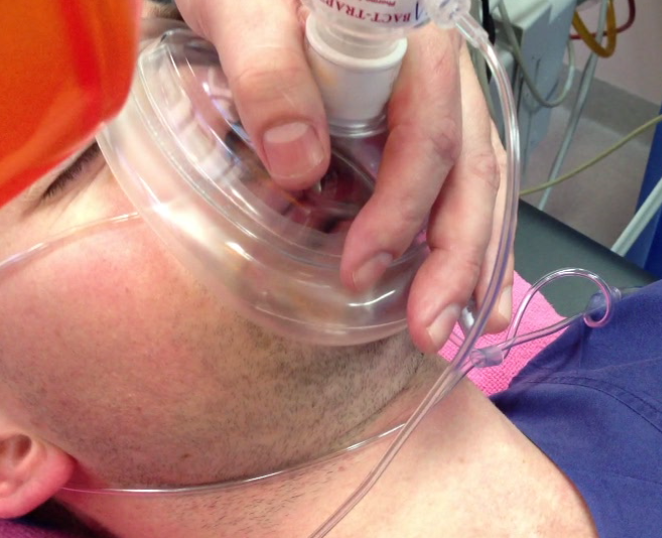
2. Firm Seal/High Flows: devices capable of delivering an FiO2 of 100% will only realise this potential if the face mask is correctly sized to fit the patient’s face and applied firmly so that there are no leaks which allow dilution of the inspired oxygen with room air. Prevention of leaks is dependent on mask design, operator technique and patient factors (e.g. more difficult with beard, edentulous). Even small leaks such as that depicted opposite can lead to a dramatic decrease in FiO2 due to entrainment of room air. In the demonstration depicted opposite this small leak resulted in end tidal oxygen concentrations of only 40% following 3 mins of preoxygenation with 100% oxygen supplied at 15L/min via an anaesthetic circle circuit. Thus preoxygenation holding the face mask above the patient’s face without any seal (as is sometimes observed in elective anaesthetic practice) allows entrainment of large volumes of room air and significantly decreases the effective inspired oxygen concentration, diminishing the effectiveness of pre- oxygenation and the safe apnoea time. This issue is exaggerated when pre-oxygenation is being performed via a bag-valve-mask device with a one way valve at the patient end to prevent rebreathing (see photo opposite), as limited oxygen will flow from the mask unless a pressure differential exists which opens the valve. This means that if the bag is held away from the patient’s face, such that a negative pressure cannot be generated by patient inspiration to open the valve, only low oxygen flows will be delivered and the patient will receive predominantly room air.
With some equipment it is possible to supply 100% O2 despite the absence of an occlusive seal if the 100% O2 source is supplied at a flow rate that exceeds maximal inspiratory flow rates and thus circumvents the entrainment of room air.
3. Adequate alveolar ventilation: washout of nitrogen and its replacement with high concentrations of oxygen being inspired during pre-oxygenation is a function of both the rate of alveolar ventilation and the duration of pre-oxygenation. Adequate pre-oxygenation is achieved with 3 mins of normal tidal ventilation. Similar degrees of pre-oxygenation can be achieved by 8 vital capacity breaths within 60 secs. The time to effective pre-oxygenation can be further reduced by encouraging cooperative patients to exhale to residual volume prior to commencing pre-oxygenation.
Volume of Functional residual capacity
In addition to the above techniques to improve the oxygen concentration in the FRC, optimal preoxygenation should involve consideration of additional strategies to increase the volume of the FRC such as PEEP/CPAP and 20 degrees head up tilt. These strategies maximise the volume of the reservoir which can be filled with high concentration oxygen and is available in the event that an interruption to alveolar oxygen delivery occurs. By limiting atelectasis and shunting of pulmonary blood these techniques also improve gas exchange, maximising the impact of both preoxygenation, reoxygenation and apnoeic oxygenation techniques.
Barriers to Preoxygenation
Absorption Atelectasis:
Some clinicians have raised concerns that inspired oxygen concentrations of 100% may cause harm by causing absorption atelectasis and have advocated using an inspired oxygen concentrations of 80% or less for pre-oxygenation. Whilst preoxygenation with 100% vs 80% oxygen has been demonstrated to produce an increased degree of atelectasis on CT studies, no causal link has been demonstrated between this radiological finding and increased pulmonary complications. In any case any airway closure resulting from use of 100% oxygen for preoxygenation is readily reversed by use of a recruitment manoeuvre (>40cm H2O for >15 sec) once a definitive airway has been established. Conversely preoxygenation with 80% oxygen results in a clinically significant decrease in the safe apnoea time. As such concerns regarding absorption atelectasis should not represent a deterrent and preoxygenation with 100% oxygen is advocated to maximise patient safety, particularly in situations where the possibility of a delay in restoring alveolar oxygen delivery &/or a more rapid desaturation with interruption to the oxygenation continuum, are anticipated.
Patient Discomfort:
“it should be very rare that full preoxygenation is not possible due to patient discomfort”
Some clinicians are reluctant to perform preoxygenation due to concerns that a tight fitting mask will cause discomfort to patients. Most patients tolerate preoxygenation well but a small proportion do experience significant distress from application of a tight fitting mask. A number of techniques are available which still allow fulll preoxygenation in these patients without causing distress:
An Entonox mouthpiece attached to a circle, Mapleson or BVM (with patient breathing only through their mouth) can be used to provide 100% oxygen for preoxygenation in patients who will not tolerate an occlusive face mask.
A cut off ETT can also be used as a makeshift mouthpiece to allow preoxygenation in patients who will not tolerate a face mask.
- Nasal preoxygenation: application of a paediatric face mask only to the nose of the patient with a tight seal may be better tolerated than a mask placed over the nose and mouth. Provided the patient is instructed to breathe in and out only through their nose this technique allows full preoxygention with 100% oxygen in the same manner as a face mask.
- Mouthpiece preoxygenation: connection of a mouthpiece (see image) or cut off endotracheal tube to the anaesthetic circuit or BVM which can be held between the lips (creating a seal) allows full preoxygenation with 100% oxygen and may be better tolerated in patient's who find an occlusive mask seal claustraphobic. It is important that the patient breaths only through their mouth when using this technique and asking the patient to hold their nose is recommended to prevent entrainment of room air.
- Non-rebreather mask: use of some designs of non-rebreather mask in combination with 15L/min supplementary oxygen via nasal prongs to compensate for the lack of a seal around the mask, can provide adequate preoxygenation and may be better tolerated by patients who are distressed by application of a tight fitting mask with an occlusive seal. The inspired oxygen concentration provided by this method may vary with different designs of non-rebreather mask so this should be confirmed with the particular devices used before using this technique.
- High Flow Humidified Nasal Oxygen (HFHNO): this technique utilises very high flow (up to 70L/min) humidified nasal oxygen. Since these flows exceed the inspiratory flow rate they allow for full preoxygenation without the entrainment of room air, despite the absence of an occlusive face mask, provided the patient does not speak and keeps their mouth closed. If patient speaks or breathes through their mouth during PreOx with HFHNO an FiO2 of 100% is not reliably achieved.
Using the above variations to the standard technique where necessary, it should be very rare that full preoxygenation is not possible due to patient discomfort.
Patient Cooperation:
Full preoxygenation may be difficult in patients who are uncooperative with having oxygen delivery devices applied for the required time. In these patients light sedation can be useful to assist with them tolerating a face mask. As patients who are acutely distressed and uncooperative also tend to unfasted, the risk of sedation and the attendant potential for impaired airway protection and pulmonary aspiration, must be weighed against the risk of interruption to the oxygenation continuum without preoxygenation. Small, titrated doses of ketamine tend to produce less risk of impairment of airway protective reflexes than other sedatives but ketamine sedation should not be considered to be without risk in this regard. The emergency medicine literature has formalised this process in terms of the technique of 'Delayed Sequence Intubation' (DSI). This technique conceptualises DSI as a form of procedural sedation in which the procedure is preoxygenation.
Time:
It is difficult to conceive of many circumstances in which there is inadequate time available to perform preoxygenation prior to advanced airway management, particularly when using the 8 vital capacity breaths technique. Time pressure should not generally be regarded as an impediment to performing preoxygenation which typically can be undertaken in parallel with other preparations for airway management.
Use of Capnography During Preoxygenation
Preoxygenation should ideally be performed with capnography attached. Whilst this is usual practice in an operating room setting using an anaesthetic machine, it may be overlooked in other settings such as the post-anaesthesia care unit (PACU), emergency department (ED) or intensive care unit (ICU) when a bag-valve-mask device or Mapleson circuit is being used. Preoxygenation with ETCO2 monitoring attached provides the following ancillary benefits:
- Confirmation of functioning capnography: having capnography attached during preoxygenation provides an opportunity to check that the capnograph is available, ready & functioning prior to induction. This avoids confusion about whether subsequent absence of an ETCO2 trace, using any of the upper airway lifelines, is due to a measurement error or an interruption to alveolar oxygen delivery.
- Confirmation of correctly fitting mask: where a waveform capnograph trace is present, inability to achieve a good square waveform may indicate a leak around the mask. This is significant on two fronts:
- Impaired preoxygenation: entrainment of room air through this leak during preoxygenation will diminish the effectiveness of denitrogenation of the alveoli and diminish safe apnoea time if there is an interruption to alveolar oxygen delivery
- Compromised mask ventilation: if an effective seal cannot be achieved with the mask during preoxyenation it is likely that this same leak will impair the ability to apply positive pressure for the purposes of mask ventilation post induction, increasing the likelihood of an interruption to alveolar oxygen delivery.
- Confirmation of effective facemask ventilation: capnography is important not just for confirming correct placement of an endotracheal tube, but also for confirming whether ventilation (& thus alveolar oxygen delivery) is occurring when other lifelines are used. Even if the primary intention is placement of an endotracheal tube, a face mask is likely to be used as the primary rescue techniques if intubation is initially unsuccessful. Having capnography already attached to the face mask allows immediate assessment of whether the Green Zone has been entered as failure to obtain an ETCO2 trace when ventilating via a facemask suggests that alveolar oxygen delivery is not occurring.
Endpoints to preoxygenation
Completion of effective pre oxygenation is typically determined one of the following criteria:
- End-Tidal Oxygen Concentration: ideally oxygen analysers capable of measuring end-tidal oxygen concentrations should be available in all settings in which advanced airway management takes place as this represents the most reliable way of assessing the adequacy of preoxygenation. Generally an end-tidal O2 (ETO2) concentration > 80% can be considered to represent full preoxygenation – but the highest achievable ETO2 should always be aimed for. While ETO2 monitoring is standard in an operating theatre environment it may not be available routinely in other settings such as PACU, ED or ICU.
- Time: where end-tidal O2 analysis is not available, preoxygenation is usually considered to be complete after 3 minutes (assuming normal tidal ventilation and a good seal) as described above.
- Number of Breaths: as described above, 8 vital capacity breaths will usually achieve full PreO2 when gas analysis is not available.
“a static saturation reading is in fact irrelevant to determining whether a patient is adequately preoxygenated”
Note that oxygen saturations are not included amongst the list of criteria to assess the adequacy of preoxygenation. A static saturation reading is in fact irrelevant to determining whether a patient is adequately preoxgenated. Since most healthy individuals have an SpO2 close to 100% when breathing 21% oxygen, a high SpO2 cannot be used to infer that the FRC contains gas with an oxygen concentration approaching 100%. Similarly, very unwell patients may have poor gas exchange and thus poor oxygen saturations despite full preoxygenation. Only a dynamic SpO2 reading is relevant to determining whether the patient is adequately preoxygenated: if the SpO2 is continuing to rise during PreO2, then the oxygen content of the blood is rising and it is reasonable to assume that the alveolar oxygen concentration is also still rising, and to continue preoxygenation until the SaO2 plateaus, even if the 3 minute time period for PreO2 has already elapsed.
References:
Hedenstierna G. Oxygen and anaesthesia: what lung do we deliver to the post-operative ward? Acta Anaesthesiol Scand 2012; 56 675-685
Chrimes N. Not all bag-valve-mask devices are created equal: beware a possible lower FiO2 during spontaneous ventilation. Anaesth Intensive Care 2014; 42(2) 276

Reoxygenation
REOX
Reoxygenation
REOX
benefits of reoxygenation
The opportunity to reoxygenate the patient arises any time the Green Zone is entered. Reoxygenation via positive pressure ventilation during airway management provides exactly the same benefits as those described using preoxygenation during spontaneous ventilation prior to airway management. Thus reoxygenation is not just an opportunity to return low oxygen saturations to normal levels but to replenish oxygen stores in the functional residual capacity (FRC) of the lung and restore the safe apnoea time in case there is a further interruption to alveolar oxygen delivery.
An ancillary benefit is that successful face mask ventilation following induction, while waiting for muscle relaxants to take effect, also provides the team with confirmation of being in the Green Zone which can decrease stress and allow airway subsequent airway management to be approached in a more controlled way. Conversely recognition of the inability to mask ventilate provides the opportunity to make the most efficient use of the safe apnoea time via early commencement of optimisation interventions according to the Vortex Approach and gives the team increased situational awareness of the significance of the inability to enter the Green Zone via other lifelines during subsequent attempts.
face mask ventilation during rapid sequence induction
Even during a rapid sequence induction (RSI), gentle face mask ventilation with inspiratory pressures <15cmH2O whilst waiting for the onset of muscle paralysis is acceptable and should not cause gastric insufflation or increase the risk of regurgitation of gastric contents. Maintaining ventilation pressures below this limit is more reliably achieved in an operating theatre setting where circuits with soft bags (that provide good tactile feedback about airway pressures), adjustable pressure limiting valves and airway manometry provide some safeguards against excessive ventilation pressures. It may be reasonable to exercise greater caution during RSI in other environments where the lack of tactile feedback about ventilation pressures from the more rigid self inflating bag of a BVM device and the absence of airway manometry place clinicians at increased risk of causing gastric insufflation with the potential to provoke regurgitation and aspiration.

Apnoeic oxygenation
APOX
Apnoeic oxygenation
APOX
PHysiology of Apnoeic oxygenation
The process of apnoeic oxygenation (ApOx) relies on the discrepancy between the rate at which oxygen is normally removed from the alveoli compared with that at which carbon dioxide (CO2) is delivered to them. Predominantly due to differences in their respective solubilities in the blood, it is estimated that during apnoea CO2 enters the alveoli at a rate of only approximately 10ml/min whilst oxygen is removed at a rate of approximately 250ml/min. This 240ml/min net removal of gas volume from the alveoli during periods of apnoea results in a reduction in barometric pressure in the alveoli. The sub-atmospheric pressure which develops serves as the driving force for gas to move from the upper airway to the alveoli by the process of apnoeic mass movement, provided the airway is patent. The mechanism for apnoeic mass movement is thus bulk flow down a pressure gradient, not molecular diffusion, yet is able to occur during periods of apnoea as it does not depend upon the generation of positive pressure in the upper airway nor on respiratory effort by the patient. This process has been recognised for over fifty years but has only recently started being used as a routine adjunctive technique to deliberately prolong the safe apnoea time in susceptible patients.
The requirements for effective apnoeic oxygenation are as thus as follows:
- High Oxygen Concentration: apnoeic oxygenation can only be effectively performed following preoxygenation to ensure high oxygen concentrations thoughout the respiratory tree. This is required to ensure that the a sufficient volume of gas is taken up from the alveoli and that this is replenished with gas from the upper airway that contains sufficient oxygen to maintain adequate oxygenation of pulmonary capillary blood and allow the process to continue. It should be noted that for these reasons apnoeic oxygenation is not effective in restoring the oxygen saturations of patients that have already desaturated - as during airway management desaturation usually indicates that a low oxygen concentration has developed in the alveoli.
Oxygen source: insufflation of high flow oxygen into the upper airway via the nasal or buccal routes prevents entrainment of room air so that further high concentration oxygen is available to move down the trachea to the alveoli and replace that being consumed.
Patent Airway: oxygen delivered to the upper airway can only move towards the lungs in response to the subatmospheric alveolar pressure if the airway is patent. A nasopharyngeal airway may be useful to assist nasal oxygen passing from the nasopharynx into the hypopharynx.
LIMITATIONS OF apnoeic Oxygenation
Although apnoeic oxygenation has been shown to prolong the safe apnoea time significantly this is not guaranteed and some patients may still rapidly desaturate despite these techniques. Apart from airway obstruction another factor limiting the efficacy of apnoeic oxygenation is alveolar collapse. Rather than draw further gas into the alveolus, the negative pressure generated there can potentially lead to loss of volume and ultimately collapse of the alveolus (aborption atelectasis). This can result in a pulmonary shunt and depression of the oxygen saturations. Patients with pre-existing shunt physiology and those pre-disposed to developing it during periods of apnoea are therefore less likely to benefit from apnoeic oxygenation techniques. The likelihood of developing absorption atelectasis in depends in part on the balance of expansive and retractive forces acting on the alveoli and the resistance to gas flow in the respiratory tree. Unfortunately morbidly obese patients in whom prolongation of the safe apnoea time might be most desirable are are more susceptible to developing shunt physiology during apnoea and may have the least benefit. Still, prolonged periods of sustained blood oxygenation have been demonstrated in obese patients using apnoeic oxygenation. Even obese patients, who desaturate more rapidly due to shunt, may still be demonstrating significant prolongation of their safe apnoea time relative to what it might have been in the absence of apnoeic oxygenation. This is supported by animal models where even though a higher shunt fraction has been associated with a more precipitous initial fall in SpO2 at the onset of apnoea (even with use of apnoeic oxygenation), the value at which the falling SpO2 has plateaued has been higher when apnoeic oxygenation has been implemented suggesting that a benefit is still being derived. Once shunt becomes sufficiently severe, however, the plateau SpO2 level will not longer be sufficiently high to qualify as 'safe'. A high shunt may be responsible for the inability to demonstrate a benefit from apnoeic oxygenation during airway management in critically ill patients.
Minimising atelectasis via application of CPAP and head up tilt should reduce shunt and maximise the impact of apnoeic oxygenation techniques.
The important point is to be aware that apnoeic oxygenation is a important adjunctive tool to extend the safe apnoea time but that it cannot be relied upon or used as an alternative to the definitive strategy of restoring alveolar oxygen delivery via one of the upper airway lifelines or CICO Rescue. Apnoeic oxygenation should be used to extend the time available to efficiently make attempts to restore alveolar oxygen delivery by these techniques without desaturation occurring but the maintenance of high saturations it provides should not influence decision making about the need to move on to alternative strategies where best efforts at one or more of the upper airway lifelines have not secured entry into the Green Zone.
"confirmed" alveolar oxygen delivery
“apnoeic mechanisms to deliver oxygen to the alveoli do not provide the information required to make timely decisions about whether this goal is being achieved and the need to optimise or move on to an alternate technique during airway management”
Effective decision making during airway management requires the ability to rapidly confirm whether alveolar oxygen delivery has been achieved by a particular strategy. This allows an immediate judgement to be made about the need to perform optimisation manoeuvres or move on to an alternate technique. The inability to promptly identify that a technique to achieve alveolar oxygen delivery has been unsuccessful wastes valuable ‘safe apnoea time’ during which rescue strategies could have be implemented and increases the risk of exposing the patient to sustained hypoxia. Apnoeic oxygenation techniques do do not allow a prospective assessment to be made of whether they are achieving alveolar oxygen delivery or how long existing oxygen saturations are likely to be maintained before desaturation occurs. Since apnoeic methods for oxygen delivery do not typically produce a rise in existing oxygen saturations, their success or failure can only be identified in hindsight by observing when oxygen saturations begin to decline. Thus from a practical perspective, apnoeic mechanisms to deliver oxygen to the alveoli do not provide the information required to make timely decisions about whether this goal is being achieved and the need to optimise or move on to an alternate technique during airway management. As such the Vortex Approach restricts the meaning of the term "alveolar oxygen delivery" to refer only to the concept of confirmed alveolar oxygen delivery. Since alveolar oxygen delivery cannot be directly measured, real-time assessment of whether it is occurring must be inferred from other clearly definable endpoints. Real-time confirmation of alveolar oxygen delivery can thus only be achieved by confirming ventilation with oxygen or direct tracheal insufflation of oxygen via a neck airway. In most circumstances this involves obtaining a capnography trace and/or evidence of an upward trend in oxygen saturations (as opposed to sustained high saturations). The ability to confirm alveolar oxygen delivery is thus restricted to those techniques which involve cyclical inflation/deflation of the lungs as the mechanism to deliver oxygen – ventilation via the upper airway lifelines or ventilation/intermittent insufflation via neck airways. Techniques involving apnoeic mechanisms for delivering oxygen (“unconfirmed” alveolar oxygen delivery) are better considered as techniques to prolong the safe apnoea time.
As such while apnoeic oxygenation, when successful, does technically achieve "alveolar oxygen delivery" the Vortex Approach emphasises the need to achieve ‘confirmed’ alveolar oxygen delivery and uses the term "alveolar oxygen delivery" in this way.
TECHNIQUES for Apnoeic Oxygenation
NO DESAT (Nasal Oxygen During Efforts Securing A Tube)
While, if the airway is patent, apnoeic oxygenation occurs in any preoxygenated patient while a face mask is applied, the NO DESAT technique allows the benefits of apnoeic oxygenation to be yielded even during attempts at laryngoscopy. This enables apnoeic oxygenation to be achieved even in many patients in whom airway patency cannot be established via the three lifelines of face mask, supraglottic airway and endotracheal tube. This is possible because even when a patent airway cannot be achieved via one of the three lifelines, in the absence of laryngeal pathology or a foreign body, it is likely that during attempts at laryngoscopy the upper airway will still be patent and able to allow delivery of oxygen to the alveoli via apnoeic mass movement – even though a view of the larynx may not be able to be achieved or an endotracheal tube passed. Thus whilst the patent airway achieved with a laryngoscope in place does not allow for positive pressure ventilation, if a reservoir of high concentration oxygen can be created in the nasopharynx it can be drawn towards the alveoli by apnoeic mass movement.
Nasal cannulae alone provide only low inspired oxygen concentrations in spontaneously ventilating patients. This occurs because even during quiet breathing the inspiratory flow rate (in contrast to the minute ventilation which is the averaged flow rate over time derived from intermittent inspiration/expiration) of gas in and out of the lung is much higher (upto 30Lt/min) than the oxygen flow rate delivered by the cannulae. The result is that significant volumes of room air must also be entrained during inspiration to make up this difference. This low oxygen concentration gas mixes with the 100% oxygen entering the nasopharynx via the nasal prongs (as well as with low oxygen concentration gas present in the anatomical dead space at the end of the previous expiration), diluting the concentration of oxygen in the gas that is drawn into the alveoli with inspiration down to around only 30% .
The effect of oxygen delivery via nasal cannulae in the apnoeic patient is quite different, however. In the absence of ventilation, no room air or expired gas with a low oxygen concentration enters the nasopharynx to dilute the 100% oxygen insufflated by the nasal prongs. The result is that if nasal cannulae are applied with flows of 15Lt/min during preoxygenation, then once apnoea occurs they provide a reservoir of near 100% oxygen in the nasopharynx which is available to be drawn down the trachea into the alveoli by apnoeic mass movement. A second oxygen oxygen source, in addition to that being used to supply the mask being used, is required to provide oxygen flow for the nasal cannula.
Using nasal cannulae at 15L/min, apnoeic oxygenation has been demonstrated to extend the duration of apnoea without desaturation to beyond 10mins in some cases.
THRIVE (Transnasal Humidified Rapid Insufflation Ventilatory Exchange)
Dr Anil Patel discusses the possibilities and limitations of THRIVE at the ANZCA ASM 2016
THRIVE employs very high flow (upto 70L/min) humidified nasal oxygen and has resulted in extension of the safe apnoea time by a mean of 14 mins and upto an hour in some patients. In addition to apnoeic oxygenation, THRIVE has the following benefits in relation to extending the safe apnoea time:
- Apnoeic Ventilation: limiting the rise in arterial CO2 levels during the apnoeic period (although high CO2's and respiratory acidosis are typically well tolerated in otherwise well patients). This has potential benefits when used in contexts of prolonged (> 15min) apnoea, such as some ENT surgery, but probably does not provide a significant advantage during emergency airway management when restoration of ventilation by other means would typically have been achieved well within this timeframe.
- Continuous positive airways pressure (CPAP): which can reduce atelectasis and shunting thereby assisting with maintenance of oxygen saturations. In addition this could assist with splinting open of the upper airway and maintenance of airway patency without use of an occlusive face mask.
- Increased FiO2 during PreOx: as the flow rates using THRIVE are able to exceed inspiratory flow rates, it can also be used for preoxygenation, without the need for an occlusive face mask, while avoiding the discomfort that occurs when these high flows are delivered using non-humidified oxygen sources (see below).
Unrelated to its impact on safe apnoea time, the warm humidified gas used in THRIVE has adjuvant benefits in decreasing discomfort (when used in awake patients) as well as limiting mucosal injury and impairment of ciliary function, with the potential to decrease the incidence of subsequent respiratory infections (compared with use of cold, dry gas).
The equipment for nasal oxygen delivery during THRIVE involves bulky corrugated tubing which prevents being able to achieve a seal with a face mask while it is in place. As such THRIVE must be removed and replaced before and after each attempt at face mask ventilation which increases the complexity associated with its use during management of a challenging airway. THRIVE also requires dedicated equipment which is more expensive in comparison to that required for NODESAT or buccal ApOx (which utilise cheap, generic airway equipment). As such it is logical to limit use of THRIVE to the circumstances in which it offers particular advantages: prolonged apnoea, high risk of atelectasis and maintenance of a high FiO2/CPAP in situations where use of an occlusive face mask is undesirable.
Buccal Oxygen Delivery
This technique provides supplementary oxygen flow via the oral route using an adapted RAE endotracheal tube secured in the left buccal space. Like NODESAT this technique allows the benefits of ApOx to be obtained during attempts at laryngoscopy and does not interfere with the ability to face mask ventilate between these attempts (unlike THRIVE - see above). It is also simpler to apply than
It has been postulated that buccal oxygen delivery may be superior to NODESAT and THRIVE because it offers additional protection from the theoretical risk of pulmonary or gastric barotrauma with these techniques (see below), since the position of the oxygen source in the oral cavity ensures that any rise in pharyngeal pressure will be vented via the mouth. In contrast it is theoretically possible for airway pressures to rise during NODESAT if the oral cavity becomes obstructed by the tongue/soft palate. Additionally it has been suggested that the efficacy of NODESAT may be limited by the occurrence of retropalatal obstruction when obese patients are induced for airway management. Supplementary oral oxygen is not impacted by obstruction of passage between the naso- & oropharynx by the soft palate, and effective ApOx using this technique requires only that airway patency between the oro- & laryngopharyx is able to be maintained by performance of sustained laryngoscopy. Buccal oxygen supplementation is also advantageous when performing ApOx in patients in whom nasal instrumentation is contraindicated.
In obese patients, ApOx using a buccal oxygen source at 10L/min has been demonstrated to significantly reduce the risk of desaturation during apnoeic episodes in excess of 12 minutes. This flow rate (which is less than that used with NODESAT) provides a high pharyngeal oxygen concentration during apnoea, allowing ApOx to occur, but probably provides negligible CPAP and does not produce the apnoeic ventilation phenomenon of THRIVE.
Concerns with nasal oxygen insufflation
Interference with Face Mask Seal: one concern with use of the NODESAT technique is the possibility that the nasal cannula oxygen tubing might interfere with getting a good seal for face mask ventilation – though in practice this is not usually an issue, and nasal cannulae could be easily removed if this were the case. The large diameter corrugated tubing used for THRIVE does prevent a face mask seal but the THRIVE nasal cannulae can be easily removed to allow face mask ventilation and replaced again during attempts at laryngoscopy. This does provide an extra task during management of a challenging airway and it is forseeable that replacing the THRIVE cannula during laryngoscopy could be overlooked resulting in a termination of apnoeic oxygenation.
Barotrauma: the pressure generated from insufflation of gas into the upper airway is a function of the rate of gas flow and the resistance flow of this gas back to the atmosphere via the upper airway. If none of the insufflated gas were able to be vented to the atmosphere it would rapidly accumulate in the stomach or lungs leading to a rise in volume/pressure and the potential for serious complications. Gastric rupture has been reported with insufflation of relatively low flows (4L/min) of oxygen via long nasal/nasopharyngeal catheters during procedural sedation but remains a only theoretical concern with use of NO DESAT & THRIVE which utilise shorter intranasal cannulae but at much higher flow rates. THRIVE has been shown to produce elevation of pulmonary pressures equivalent to only 5cmH2O but this limit is dependent on the upper airway remaining unobstructed to allow venting of insufflated gas. Supplementary oxygen via the buccal route has been postulated to be safer a safer technique for ApOx by providing a reliable, low resistance route for egress of insufflated oxygen and avoiding this theoretical risk.
ancilliary benefits of Supplementary oxygen insufflation
In addition to allowing apnoeic oxygenation there are a number of potential ancillary benefits from using supplementary insufflation of oxygen during preoxygenation and airway management.
- Increased FiO2: the supplementary oxygen flow can compensate for room air entrainment which would otherwise prevent some oxygen delivery devices (e.g. a non-rebreather mask) providing sufficient inspired oxygen concentrations to allow for preoxygenation in spontaneously ventilating patient. Utilising supplementary nasal or buccal oxygen for this indication requires a clear understanding of the oxygen delivery capabilities of the specific devices when used in this way as the achievement of an adequate FiO2 for preoxygention is not guaranteed. Use of non-humidified gas at high flow rates during these techniques may also cause significant discomfort to fully conscious patients. Utilising an anaesthetic circuit (circle or Mapleson) or a Bag-Valve-Mask device with an expiratory port valve that provides the ability to achieve a face mask seal is preferred wherever feasible.
- Leak Compensation: supplementary oxygen flow via nasal or buccal routes may compensate for the loss of gas via small face mask leaks and improve the ability to achieve effective positive pressure ventilation via face mask in these circumstances.
- PEEP/CPAP: supplementary oxygen flow using nasal cannulae for NODESAT can assist in generating CPAP/PEEP thereby limiting atelectasis, reducing intrapulmonary shunting and optimising oxygenation of pulmonary capillary blood. In addition PEEP/CPAP increases the volume of the functional residual capacity and thus the volume of the oxygen reservoir generated during preoxygenation.
- Splinting of upper airway: based on anecdotal observation it is possible that nasal (but not buccal) oxygen flow could act to splint open the upper airway in effect creating a "pneumatic nasopharyngeal airway" that improves the ease of face mask ventilation by holding the soft palate open. This has not been confirmed in clinical trials. Conversely, as mentioned above, persistent obstruction of the nasopharynx by the soft palate, preventing nasally insufflated oxygen reaching the respiratory tree, has also been proposed as one of the factors potentially limiting the effectiveness of supplementary nasal oxygen in prolonging the safe apnoea time in obese patients (and thus an advantage of buccal oxygen supplementation).
References:
Levitan RM. NO DESAT! Nasal oxygen during efforts securing a tube. Emergency Physicians Monthly. December 9, 2010. www.epmonthly.com/?features/?current-features/?no-desat-/?. Accessed August 3, 2016.
Frumin MJ, Epstein RM, Cohen G. Apneic Oxygenation in Man. Anesthesiology 1959; 20(6): 789-98
Teller LE, Alexander CM, Frumin MJ, Gross JB. Pharyngeal Insufflation of Oxygen Prevents Arterial Desaturation during Apnea. Anesthesiology 1988; 69: 980-82
Patel A, Nouraei SAR. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015; 70: 323–329
Patel A, Nouraei SAR. Nasal Ventilation: Oxygenation, NO DESAT, and THRIVE. Anesthesiology News. August 8th 2016. Available from http://www.anesthesiologynews.com/Review-Articles/Article/08-16/Nasal-Ventilation-Oxygenation-NO-DESAT-and-THRIVE/37294. Accessed August 16th, 2016
Heard A, Toner AJ, Evans JR, Palacios AMA, Lauer S. Apneic Oxygenation During Prolonged Laryngoscopy in Obese Patients: A Randomised, Controlled Trial of Buccal RAE Tube Oxygen Administration. Anaesth Analg. DOI: 10.1213/ANE.0000000000001564

Conserve Oxygenation
CONOX
Conserve Oxygenation
CONOX
Decreasing oxygen consumption
Techniques which decrease oxygen consumption allow more effective use to be made of the oxygen reserves during periods of apnoea thereby conserving oxygenation. In particular, skeletal muscle fasciculations caused by suxamethonium have been shown to result in an increase in oxygen consumption of nearly 10% in animal models. Minimising or avoiding these fasciculations can prolong the time to critical desaturation. This can be achieved by the following techniques:
- Use of adjuvants to attenuate fasciculations: use of fentanyl & lignocaine during intubation has been shown to decrease both the intensity of fasciculations seen with suxamethonium use and cause a significant increase in the safe apnoea time. These drugs also cause a decrease in the mean arterial pressure and heart rate seen during intubation compared with use of suxamethonium alone, which could also potentially contribute to a decrease in whole body oxygen consumption.
- Use of rocuronium instead of suxamethonium: provides a small additional prolongation of the safe apnoea time compared with use of suxamethonium and adjuvant drugs by avoiding the occurrence of fasciculations entirely.
References:
Tang L, Li S, Huang S, Ma H, Wang Z. Desaturation following rapid sequence induction using succinylholine vs. rocuronium in overweight patients. Acta Anaesthesiol Scand 2011; 55: 203-208
Taha SK, El-Kathib MF, Baraka AS, Haidar YA, Abdallah FW, Zbeidy RA, Siddik-Sayyid SM. Effect of suxamethonium vs rocuronium on onset of oxygen desaturation during apnoea following rapid sequence induction. Anaesthesia 2010; 65: 358-361