Prinicples of preoxygenation
Preoxygenation is a safe, simple and effective technique to increase the safe apnoea time. Optimal preoxygenation requires attention to three key elements:
- Maximising the oxygen concentration in the lung functional residual capacity (FRC).
- Maximising the volume of the lung FRC
- Optimising gas exchange from the oxygenated alveoli
The first two elements increase the oxygen content in the FRC which can be used to sustain blood oxygenation in the event that alveolar oxygen delivery is interrupted. The third element improves uptake of alveolar oxygen into the blood and decreases shunt in order to maximise the blood oxygenation that can be achieved from these alveolar oxygen stores.
Alveolar oxygen concentration
Effective preoxygenation with 100% oxygen increases the oxygen content of gas in the patient’s FRC from 21% towards 100% which should theoretically produce a proportionate increase in the safe apnoea time. Whilst in practice achieving alveolar oxygen concentrations of 100% is not possible, increasing the end-tidal oxygen concentration to levels of 80-90% is usually readily achievable. The requirements for maximising alveolar oxygen concentration are as follows:
1. High Oxygen Concentration Device: despite being connected to a wall outlet or cylinder which provides a source of 100% oxygen, not all oxygen delivery devices are able to supply this 100% oxygen to the patient undiluted.
- Oxygen delivery devices such as an anaesthetic circuit (circle, Mapleson, etc) or some bag-valve-mask devices, are capable of providing close to 100% oxygen due to the presence of a reservoir bag and valves as well as a mask which can create a tight seal on the face. Together these factors prevent rebreathing/entrainment of room air allowing the device to deliver undiluted 100% oxygen to the patient.
- Whilst it is typically well appreciated that oxygen delivery devices such as non-rebreather masks, Hudson masks and nasal prongs are unable to deliver a high oxygen concentration to the patient, there is less awareness that even some designs of bag-valve-mask (BVM) device (those without an expiratory port valve), are able to deliver an oxygen concentrations of only 60% during spontaneous ventilation due to dilution of the 100% oxygen source by room air. During positive pressure ventilation BVM's will deliver 100% irrespective of whether they have an expiratory port valve. Thus BVM's without an expiratory port valve are suitable for reoxygenation of an apnoeic patient (positive pressure ventilation) but not preoxygenation (spontaneous ventilation).
- Even BVM's with an expiratory port valve may entrain room air if minute ventilation is high enough to exceed the flow of 100% oxygen supplying the device. This can lead to a dramatic fall in inspired oxygen concentration and occurs independently of whether the device is being used during spontaneous or positive pressure ventilation.
- Use of supplementary oxygen sources can compensate for the inability of some oxygen delivery devices to provide a closed system and increase the FiO2 provided by them towards acceptable levels for preoxygenation.
This video explains the reasons for the variability in the oxygen concentration provided by an array of devices and demonstrates the FiO2 achieved with them under a variety of conditions. These results are summarised in the accompanying table.
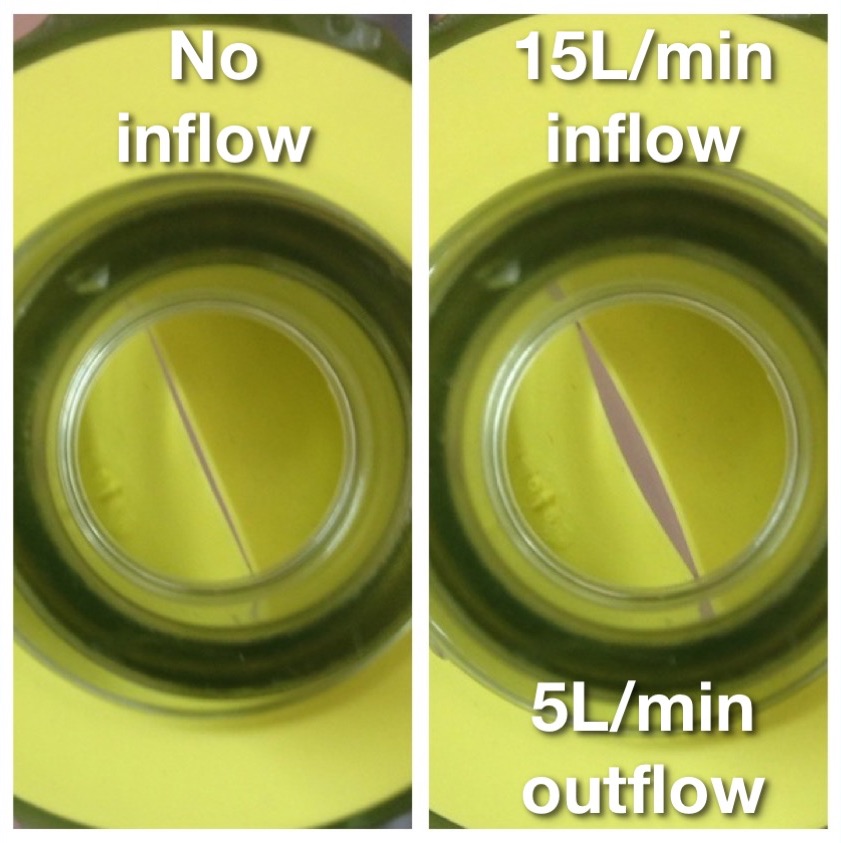
2. Firm Seal/High Flows: devices capable of delivering an FiO2 of 100% will only realise this potential if the face mask is correctly sized to fit the patient’s face and applied firmly so that there are no leaks which allow dilution of the inspired oxygen with room air. Prevention of leaks is dependent on mask design, operator technique and patient factors (e.g. more difficult with beard, edentulous). Even small leaks such as that depicted opposite can lead to a dramatic decrease in FiO2 due to entrainment of room air. In the demonstration depicted opposite this small leak resulted in end tidal oxygen concentrations of only 40% following 3 mins of preoxygenation with 100% oxygen supplied at 15L/min via an anaesthetic circle circuit. Thus preoxygenation holding the face mask above the patient’s face without any seal (as is sometimes observed in elective anaesthetic practice) allows entrainment of large volumes of room air and significantly decreases the effective inspired oxygen concentration, diminishing the effectiveness of pre- oxygenation and the safe apnoea time. This issue is exaggerated when pre-oxygenation is being performed via a bag-valve-mask device with a one way valve at the patient end to prevent rebreathing (see photo opposite), as limited oxygen will flow from the mask unless a pressure differential exists which opens the valve. This means that if the bag is held away from the patient’s face, such that a negative pressure cannot be generated by patient inspiration to open the valve, only low oxygen flows will be delivered and the patient will receive predominantly room air.
With some equipment it is possible to supply 100% O2 despite the absence of an occlusive seal if the 100% O2 source is supplied at a flow rate that exceeds maximal inspiratory flow rates and thus circumvents the entrainment of room air.
3. Adequate alveolar ventilation: washout of nitrogen and its replacement with high concentrations of oxygen being inspired during pre-oxygenation is a function of both the rate of alveolar ventilation and the duration of pre-oxygenation. Adequate pre-oxygenation is achieved with 3 mins of normal tidal ventilation. Similar degrees of pre-oxygenation can be achieved by 8 vital capacity breaths within 60 secs. The time to effective pre-oxygenation can be further reduced by encouraging cooperative patients to exhale to residual volume prior to commencing pre-oxygenation.
Volume of Functional residual capacity
In addition to the above techniques to improve the oxygen concentration in the FRC, optimal preoxygenation should involve consideration of additional strategies to increase the volume of the FRC such as PEEP/CPAP and 20 degrees head up tilt. These strategies maximise the volume of the reservoir which can be filled with high concentration oxygen and is available in the event that an interruption to alveolar oxygen delivery occurs. By limiting atelectasis and shunting of pulmonary blood these techniques also improve gas exchange, maximising the impact of both preoxygenation, reoxygenation and apnoeic oxygenation techniques.
Barriers to Preoxygenation
Absorption Atelectasis:
Some clinicians have raised concerns that inspired oxygen concentrations of 100% may cause harm by causing absorption atelectasis and have advocated using an inspired oxygen concentrations of 80% or less for pre-oxygenation. Whilst preoxygenation with 100% vs 80% oxygen has been demonstrated to produce an increased degree of atelectasis on CT studies, no causal link has been demonstrated between this radiological finding and increased pulmonary complications. In any case any airway closure resulting from use of 100% oxygen for preoxygenation is readily reversed by use of a recruitment manoeuvre (>40cm H2O for >15 sec) once a definitive airway has been established. Conversely preoxygenation with 80% oxygen results in a clinically significant decrease in the safe apnoea time. As such concerns regarding absorption atelectasis should not represent a deterrent and preoxygenation with 100% oxygen is advocated to maximise patient safety, particularly in situations where the possibility of a delay in restoring alveolar oxygen delivery &/or a more rapid desaturation with interruption to the oxygenation continuum, are anticipated.
Patient Discomfort:
“it should be very rare that full preoxygenation is not possible due to patient discomfort”
Some clinicians are reluctant to perform preoxygenation due to concerns that a tight fitting mask will cause discomfort to patients. Most patients tolerate preoxygenation well but a small proportion do experience significant distress from application of a tight fitting mask. A number of techniques are available which still allow fulll preoxygenation in these patients without causing distress:
An Entonox mouthpiece attached to a circle, Mapleson or BVM (with patient breathing only through their mouth) can be used to provide 100% oxygen for preoxygenation in patients who will not tolerate an occlusive face mask.
A cut off ETT can also be used as a makeshift mouthpiece to allow preoxygenation in patients who will not tolerate a face mask.
- Nasal preoxygenation: application of a paediatric face mask only to the nose of the patient with a tight seal may be better tolerated than a mask placed over the nose and mouth. Provided the patient is instructed to breathe in and out only through their nose this technique allows full preoxygention with 100% oxygen in the same manner as a face mask.
- Mouthpiece preoxygenation: connection of a mouthpiece (see image) or cut off endotracheal tube to the anaesthetic circuit or BVM which can be held between the lips (creating a seal) allows full preoxygenation with 100% oxygen and may be better tolerated in patient's who find an occlusive mask seal claustraphobic. It is important that the patient breaths only through their mouth when using this technique and asking the patient to hold their nose is recommended to prevent entrainment of room air.
- Non-rebreather mask: use of some designs of non-rebreather mask in combination with 15L/min supplementary oxygen via nasal prongs to compensate for the lack of a seal around the mask, can provide adequate preoxygenation and may be better tolerated by patients who are distressed by application of a tight fitting mask with an occlusive seal. The inspired oxygen concentration provided by this method may vary with different designs of non-rebreather mask so this should be confirmed with the particular devices used before using this technique.
- High Flow Humidified Nasal Oxygen (HFHNO): this technique utilises very high flow (up to 70L/min) humidified nasal oxygen. Since these flows exceed the inspiratory flow rate they allow for full preoxygenation without the entrainment of room air, despite the absence of an occlusive face mask, provided the patient does not speak and keeps their mouth closed. If patient speaks or breathes through their mouth during PreOx with HFHNO an FiO2 of 100% is not reliably achieved.
Using the above variations to the standard technique where necessary, it should be very rare that full preoxygenation is not possible due to patient discomfort.
Patient Cooperation:
Full preoxygenation may be difficult in patients who are uncooperative with having oxygen delivery devices applied for the required time. In these patients light sedation can be useful to assist with them tolerating a face mask. As patients who are acutely distressed and uncooperative also tend to unfasted, the risk of sedation and the attendant potential for impaired airway protection and pulmonary aspiration, must be weighed against the risk of interruption to the oxygenation continuum without preoxygenation. Small, titrated doses of ketamine tend to produce less risk of impairment of airway protective reflexes than other sedatives but ketamine sedation should not be considered to be without risk in this regard. The emergency medicine literature has formalised this process in terms of the technique of 'Delayed Sequence Intubation' (DSI). This technique conceptualises DSI as a form of procedural sedation in which the procedure is preoxygenation.
Time:
It is difficult to conceive of many circumstances in which there is inadequate time available to perform preoxygenation prior to advanced airway management, particularly when using the 8 vital capacity breaths technique. Time pressure should not generally be regarded as an impediment to performing preoxygenation which typically can be undertaken in parallel with other preparations for airway management.
Use of Capnography During Preoxygenation
Preoxygenation should ideally be performed with capnography attached. Whilst this is usual practice in an operating room setting using an anaesthetic machine, it may be overlooked in other settings such as the post-anaesthesia care unit (PACU), emergency department (ED) or intensive care unit (ICU) when a bag-valve-mask device or Mapleson circuit is being used. Preoxygenation with ETCO2 monitoring attached provides the following ancillary benefits:
- Confirmation of functioning capnography: having capnography attached during preoxygenation provides an opportunity to check that the capnograph is available, ready & functioning prior to induction. This avoids confusion about whether subsequent absence of an ETCO2 trace, using any of the upper airway lifelines, is due to a measurement error or an interruption to alveolar oxygen delivery.
- Confirmation of correctly fitting mask: where a waveform capnograph trace is present, inability to achieve a good square waveform may indicate a leak around the mask. This is significant on two fronts:
- Impaired preoxygenation: entrainment of room air through this leak during preoxygenation will diminish the effectiveness of denitrogenation of the alveoli and diminish safe apnoea time if there is an interruption to alveolar oxygen delivery
- Compromised mask ventilation: if an effective seal cannot be achieved with the mask during preoxyenation it is likely that this same leak will impair the ability to apply positive pressure for the purposes of mask ventilation post induction, increasing the likelihood of an interruption to alveolar oxygen delivery.
- Confirmation of effective facemask ventilation: capnography is important not just for confirming correct placement of an endotracheal tube, but also for confirming whether ventilation (& thus alveolar oxygen delivery) is occurring when other lifelines are used. Even if the primary intention is placement of an endotracheal tube, a face mask is likely to be used as the primary rescue techniques if intubation is initially unsuccessful. Having capnography already attached to the face mask allows immediate assessment of whether the Green Zone has been entered as failure to obtain an ETCO2 trace when ventilating via a facemask suggests that alveolar oxygen delivery is not occurring.
Endpoints to preoxygenation
Completion of effective pre oxygenation is typically determined one of the following criteria:
- End-Tidal Oxygen Concentration: ideally oxygen analysers capable of measuring end-tidal oxygen concentrations should be available in all settings in which advanced airway management takes place as this represents the most reliable way of assessing the adequacy of preoxygenation. Generally an end-tidal O2 (ETO2) concentration > 80% can be considered to represent full preoxygenation – but the highest achievable ETO2 should always be aimed for. While ETO2 monitoring is standard in an operating theatre environment it may not be available routinely in other settings such as PACU, ED or ICU.
- Time: where end-tidal O2 analysis is not available, preoxygenation is usually considered to be complete after 3 minutes (assuming normal tidal ventilation and a good seal) as described above.
- Number of Breaths: as described above, 8 vital capacity breaths will usually achieve full PreO2 when gas analysis is not available.
“a static saturation reading is in fact irrelevant to determining whether a patient is adequately preoxygenated”
Note that oxygen saturations are not included amongst the list of criteria to assess the adequacy of preoxygenation. A static saturation reading is in fact irrelevant to determining whether a patient is adequately preoxgenated. Since most healthy individuals have an SpO2 close to 100% when breathing 21% oxygen, a high SpO2 cannot be used to infer that the FRC contains gas with an oxygen concentration approaching 100%. Similarly, very unwell patients may have poor gas exchange and thus poor oxygen saturations despite full preoxygenation. Only a dynamic SpO2 reading is relevant to determining whether the patient is adequately preoxygenated: if the SpO2 is continuing to rise during PreO2, then the oxygen content of the blood is rising and it is reasonable to assume that the alveolar oxygen concentration is also still rising, and to continue preoxygenation until the SaO2 plateaus, even if the 3 minute time period for PreO2 has already elapsed.
References:
Hedenstierna G. Oxygen and anaesthesia: what lung do we deliver to the post-operative ward? Acta Anaesthesiol Scand 2012; 56 675-685
Chrimes N. Not all bag-valve-mask devices are created equal: beware a possible lower FiO2 during spontaneous ventilation. Anaesth Intensive Care 2014; 42(2) 276